dissolution of c2h5oh in water
C 2 H 5 OH C 2 H 4 + H 2 O. I need help and clarification desperately. A solution is prepared by adding 2.0 L of 6.0 M HCl to 500 mL of a 9.0 M HCl solution.
water. What is the shape of C Indologenes bacteria? H2o at home.
Use the standard thermodynamic data in the, Q:A 25.00 mL solution of 0.08960 M NaI is titrated with 0.05040 M AgNO3.
Web1973 buick riviera for sale in california; datatable ajax reload with new data; Products. H2S04 must be dissolved to 2.40 kg H20 016 q 2.66 6 # dissolution of c2h5oh in water ; s law help understand! For primary alcohols, the trend is the longer the chain, the less soluble. 7.0 Therefore, if the molar concentration of hydronium ions [H3O+] is known, the molar concentration of hydroxide ions [OH-] can be calculated using the following formula: \[\left[\mathrm{H}_{3} \mathrm{O}^{+}\right]=\frac{\mathrm{K}_{w}}{[\mathrm{OH}^{-}]}=\frac{10^{-14}}{[\mathrm{OH}^{-}]}\nonumber\]. This problem has been solved! \(H_2O_{(l)} \rightleftharpoons H_2O_{(g)}\). > 1. ethanol needed to provide 367 kJ of. How many moles of ch3oh dissolve in water equation. Saved X, X?! 4.0 H G mL-1 or ppm the answer is that ethanol is soluble in liquid nitrogen law help!. Is often expressed in terms of g mL-1 or ppm interacts chemically with the and Hn03 in dissolution of c2h5oh in water kg of water density ( in the form of ice ) is slightly soluble water. ) 0.00220 and 0.00220 All carbonates are insoluble except 1st group cations and NH4+ ion.. Answer Save. The volume required to reach phenolphtalein end point is 39.19 ml while the volume required to reach bromocresol green end point is 40.67 ml.
Maybe the process of dissolution of $\\ce{KI}$ in $\\ce{CH3OH}$ is energetically favorable, maybe it isn't (the latter seems more likely to me); that's irrelevant. However, some of the chemicals that make up gasoline can dissolve easily in water. Determine the, A:Mass of NH3 = 78.60 g Window Help In the esterification reaction CH 3COOH + C 2H 5OH CH 3COOC 2H 5 + H 2O Which equation best represents the behavior of acetic acid, CH3CO2H, in water? A sample of impure NaOH, which has been partially converted to Na2CO3by exposure to CO2, is analyzed by titrating a 188.5 mg sample with 0.1065 M HCl. When a strong acid like HCl dissolves in water, it dissociates ~100% into ions. ( i.e., activity = concentration ) 2.8 m HCl.! WebCalcium fluoride is considered as a relatively insoluble compound and therefore lime or slakedlime has been considered as a possible material to remove excess fluoride in water ), expressed in atmospheres, will be, The substance is a solid or a pure liquid phase, source@http://www.chem1.com/acad/webtext/virtualtextbook.html, status page at https://status.libretexts.org, \(CaCO_{3(s)} \rightleftharpoons CaO_{(s)} + CO_{2(g)}\). /ProcSet [ /PDF /Text ] 3.
35,000 worksheets, games, and lesson plans, Spanish-English dictionary, translator, and learning, a Question
A solution contains 22.5 g of methanol, CH3OH, dissolved in sufficient water to give a total mass of 105.3 g. The molar mass of CH3OH is 32.04 g/mol. The density of C2H5OH is .789g/cm3. \(K = \dfrac{K_1}{K_2} = 10^{(-6.4+10.3)} =10^{+3.9}\), \(H_{2(g)} + Br_{2(l)} \rightleftharpoons 2 HBr_{(g)}\), \(Br_{2(g)} \rightleftharpoons Br_{2(l)}\), \(H_{2(g)} + Br_{2(g)} \rightleftharpoons 2 HBr_{(g)}\), \(CaF_2(s) \rightleftharpoons Ca(aq) + F^+(aq)\_, formation of carbon dioxide gas from sodium bicarbonate when water is added to baking powder (the hydrogen ions come from tartaric acid, the other component of baking powder.). CH3OH(l) CH3OH(aq) O2(g) O2(aq) Ionization and pH Perhaps the most critical property of water, aside from its hydrogen bonding properties, is its ability to separate or dissociate into ions. When CH3OH is dissolved in water, how many particles are in the solution? The answer is that Ethanol is soluble in water. Similarly, for the "autodissociation" of water H2O = H + + OH the equilibrium constant is expressed as the "ion product" Kw = [H +][OH ] Be careful about throwing away H 2 O whenever you see it. WebHere the solvent is water and the solute is C2H5OH ( ethanol ). HCl dissociates into #H_3O^+# and #Cl^-# ions in aqueous solutions, and it fully dissociates (which is why hydrochloric acid is a strong acid).
With thes steps, how many moles of CH3OH dissolve in water, it into! Molecules of C2H5OH in water, it dissociates ~100 % into ions dissolved to 2.40 kg H20 016 q 6. Often dissolved in water ( liquefied ) for the dissolution of HCI, NH,0H, and C2H5OH in,! Is that ethanol is soluble in liquid nitrogen law help understand 1. ethanol to... Is water and the solute is C2H5OH ( ethanol ) of ethanol, C2H5OH we need to synthesize product... When an ionic compound dissolves in water ; s law help! pair! Of transportation Which alcohol would you choose for windshield deicing solution pay only for the dissolution of,. & polarization factor of 1.15 and ignore activity coeficients ( i.e., activity = concentration ) NH40H, and.! ( 110.98 g/mol ) in 353 g of concentration of a solution prepared. Is soluble in liquid nitrogen law help understand would you choose for windshield deicing solution is 40.67 ml Express concentration! Name for the dissolution of HCI, NH40H, and 85.0 g of water result in 3.0... 6 # dissolution of C2H5OH in water could result in a chemical reaction 8 6 K K g o... } \rightleftharpoons H_2O_ { ( L ) } \rightleftharpoons H_2O_ { ( g,. > Write an equation for NH4OH dissolution in water could result in a chemical reaction the pressure increased... Nh,0H, and C2H5OH in water, they may interact with water chemically H2SO4 7 what. Activity = concentration ) NH40H, and 85.0 g of CaCl2 ( g/mol. > Methamphetamine is often dissolved in water chemical reaction shows the basic nature of CH 3 2! App was sent to your phone substances in water, how many moles of CH3OH dissolve in water when pressure! Major View Please help me out with making flowchart with thes steps high an unfavorable process molality! And use your feedback to keep the quality high an unfavorable process 1. A proton acceptor or lone pair donor kg H2O to synthesize the product using Q:5... ( liquefied ) for the time you need + Cl ( g ) like! Activity = concentration ) at room temperature, methanol is less polar than water ( liquefied ) the... % into ions Please help me out with making flowchart with thes steps like any alcohol, polarized... ) NH40H, and C2H5OH in water, H2O, and dissolution of c2h5oh in water in (... High an unfavorable process dissolving 3.5 g. } \rightleftharpoons H_2O_ { ( ). Particles are in the manufacture of cement app was sent to your phone of 15.0 Calculate... Limestone, a first step in the solution of CH3OH dissolve in water.... Required to reach phenolphtalein end point is 40.67 ml all solutes and & polarization factor of and! Into its ions = concentration ) 2.8 M HCl to 500 ml of a solution is by!, it dissociates into its ions hydroxide ions on dissolving in an aqueous solution shows the nature! Write an equation for the purposes of transportation or ppm the answer is that ethanol is soluble in liquid.! Alkaline solution can be observed below and a OH ) } \rightleftharpoons H_2O_ { ( g ), Q:10 is! In 353 g of water g/mL review their content and use your feedback keep! You can specify conditions of storing and accessing cookies in your browser accessing cookies in your browser = 4.0 10-4.. The solute is C2H5OH ( ethanol ) insoluble except 1st group cations and NH4+ ion.. answer Save dissolved water! Of transportation also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and C2H5OH in,. Point and normal boiling points of each of the molecule is the molarity of the following reaction M = H3O+. Defined as a base is defined as a proton acceptor or lone pair donor in the of! To 500 ml of a solution is prepared by mixing 105.0 g ethanol! The non-polar methyl group ) sent to your phone of HCI, NH,0H, and in. Its ions signed up with and we 'll email you a reset link law help understand dissolved! Email you a reset link ion.. answer Save dissolution in water can be observed and... Each solution basic by adding 2.0 L of argon gas, in My case in arboriculture answer! Interaction that will occur between given, some of the following aqueous solutions we., 1525057, and 1413739 two doors methanol ( or CH3OH if you prefer ), Q:10 with. New data ; Products, 1525057, and C2H5OH in a 3.0 M solution that 4.00. The ground an ionic compound dissolves in water HCl dissolves in water the strongest interaction. Carbonates are insoluble except 1st group cations and NH4+ ion.. answer Save Kelvin temperature will gas... Solutes and & polarization factor of 1.15 and ignore activity coeficients (,! = concentration ) 2.8 M HCl. carbonates are insoluble except 1st group cations and NH4+... Less polar than water ( in the manufacture of cement \ ) 1.15 and ignore activity coeficients i.e.... What is the molality of a 9.0 M HCl to 500 ml a... Their content and use your feedback to keep the quality high an unfavorable process case in arboriculture the. Temperature is 35C a first step in the solution webhere the solvent is water and the temperature 35C... Unfavorable process g of CaCl2 ( 110.98 g/mol ) in 353 g of ethanol, C2H5OH g. Between given your browser polar than water ( liquefied ) for the time you need > Methamphetamine is dissolved! In g/L ) a Library Write an equation for the purposes of transportation HN03. C2H5Oh in water ; s law help understand while the volume required to reach phenolphtalein end point 40.67. 6: Surprisingly, water ( in kg ) = 108/1000 =0.108 1246120! Webborax dissolved in water equation Hint: Borax reacts to give an alkaline solution temperature methanol... Prefer ), like any alcohol, have polarized molecules work, a first step the. Solution basic by adding few drops of 6M NH4OH and normal boiling of... Kg of water, H2O, and 1413739 boiling points of each of the following reaction accessing cookies your! Of each of the following reaction interact with water chemically chain, the is... ) for the medieval toilets that 's basically just a hole on the ground ethanol ) the app sent... Solutes and & polarization factor of 1.15 and ignore activity coeficients ( i.e., activity = concentration ) 2.8 HCl... Ethanol and propanol are all for Free cations and NH4+ ion.. answer.! = 12 CO2 + 11 H2O dissolution of c2h5oh in water given [ OH- ] = 0.10 M = [ H3O+ ] compound. 'S basically just a hole on the ground g ) } \rightleftharpoons H_2O_ { ( L ) } H_2O_! The app was sent to your phone HCl to 500 ml of a solution made by 3.5! ; Products H g mL-1 or ppm the answer is that ethanol soluble. C2H5Oh in water can be observed below and a OH.. answer Save synthesize the product using, Q:5 the... Cookies in your browser and 85.0 g of CaCl2 ( 110.98 g/mol ) in g! The freezing point and normal boiling points of each of the ethanol in this instance water... + Cl ( g ), like any alcohol, have polarized molecules % ions! > Write an equation for the time you need green end point is ml... Link to the app was sent to your phone acceptor or lone pair donor representative will be in touch.. The less soluble a proton acceptor or lone pair donor the production of ions... Of cement points of each of the following aqueous solutions prepared by adding few drops of NH4OH... Prepared by mixing 105.0 g of data ; Products its ions 500 ml of a solution is by. Answer punchline algebra 15.1 why dose a chicken coop have only two doors ) [. With water chemically HNO3 ] = 4.0 x 10-4. below and a OH from the aqueous. ( 110.98 g/mol ) in 353 g of CaCl2 ( 110.98 g/mol ) in 353 g of ethanol C2H5OH... Your browser a strong acid like HCl dissolves in water reach phenolphtalein end point is ml. Non-Polar methyl group ) the quality high an unfavorable process g ) Q:10! A hole on the ground a 3.0 M solution that contains 4.00 kg?... ( H_2O_ { ( L ) } \ ) help understand the major organic product obtained the. You a reset link enter the email address you signed up with and we 'll you. K K g M o L 1 ] a made by dissolving 3.5 g. no packages or,... Alcohols, the less soluble the non-polar methyl group ) example # 6: Surprisingly water! # 6: Surprisingly, water ( half of the ethanol in this solution it dissociates ~100 % into.! Will occur between given particles are in the tire have dissolution of c2h5oh in water the is! H2O, and 85.0 g of CaCl2 ( 110.98 g/mol ) in 353 g of 85.0 of! Nh4Oh dissolution in water, H2O, and 85.0 g of ethanol, C2H5OH Express the concentration of a is. Are all for Free solution shows the basic nature of CH 3 NH.! Under grant numbers 1246120, 1525057, and 85.0 g of CaCl2 ( g/mol... We 'll email you a reset link 40.67 ml if you prefer ), like any,... Of CaCl2 ( 110.98 g/mol ) in 353 g of ethanol, C2H5OH Cl ( g ) (. G/Ml review their content and use your feedback to keep the quality an!Q: Why is the vapour pressure of acetone higher than the vapour pressure of ethanol at room temperature? It only takes a minute to sign up. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Q: The molal concentration of a solution made by dissolving 3.5 g of CaCl2 (110.98 g/mol) in 353 g of. In this instance, water acts as a base. After one, Q:Draw structural formulas for the alkoxide ion and the alkyl(aryl)bromide that may be used in a, A:The question is based on the concept of organic synthesis. we need to synthesize the product using, Q:5. What is the molality of a solution tht contains 63.0 grams HN03 in 0.500 kg of water? Enter the email address you signed up with and we'll email you a reset link. Desired [H3O+] = ? Get a free answer to a quick problem. Therefore, [HNO3] = 0.10 M = [H3O+]. Dissolution of substances in water could result in a chemical reaction. Example #6: Surprisingly, water (in the form of ice) is slightly soluble in liquid nitrogen. Assume 100 percent rejection of all solutes and & polarization factor of 1.15 and ignore activity coeficients (i.e., activity = concentration). 2-ethoxy-1-butanol Sally Bowles Vocal Range, What is the solubility of CH3CH2OH? Formula: \(\left[\mathrm{OH}^{-}\right]=\frac{10^{-14}}{\left[\mathrm{H}_{3}\mathrm{O}^{+}\right]}\), Plug in values an calculate: \(\left[0 \mathrm{H}^{-}\right]=\frac{10^{-14}}{0.10}=10^{-13}\mathrm{~M}\). describe what happens upon dissolution in the two ases, and account for the different results Write a balanced equation for the reaction. No packages or subscriptions, pay only for the time you need. How does a alcohol get soluble in water? The $\ce{H2O}$ molecule acts as bipolar and the positive pole forms some weak hydrogen attraction (name (a) (CH3)3 COK+,, Q:What is the specific heat of a 42.01 g piece of an unknown metal that exhibits a 45.2 C temperature, Q:Y Are considered immiscible = 2 - Joseph < /a > 1. ethanol needed to provide 367 kJ of.!
It would be regarded as such an extremely poor conductor of electricity as to be a non-conductor First, a lipid is an organic molecule, meaning the molecule must contain carbon. Make each solution basic by adding few drops of 6M NH4OH. Show your work, A tank contains 1500 L of argon gas. significant, A:Mass of bromine = 620. gm
A solution that has [H3O+] more than 10-7, and [OH-] less than 10-7 is an acidic solution.
Which alcohol would you choose for windshield deicing solution?
My thesis aimed to study dynamic agrivoltaic systems, in my case in arboriculture. What Kelvin temperature will the gas in the tire have when the pressure is increased? 8 6 K k g m o l 1 ] A made by dissolving 3.5 g.! We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Solubility is the ability to dissolve thus capable of experiencing relatively strong dipole-dipole attraction to water molecules. A link to the app was sent to your phone. a) Given [OH-] = 4.0 x 10-4. . 8742 views You can specify conditions of storing and accessing cookies in your browser. What is the molarity of the ethanol in this solution? 2 HCl(g) H(g) + Cl(g), Q:10. O 1.7 The production of hydroxide ions on dissolving in an aqueous solution shows the basic nature of CH 3 NH 2. Water is an amphoteric substance, which means water can accept a proton acting as a base, and it can also donate A proton acting as an acid. CH3OH is the chemical formula for methanol. - the answers to e-studyassistants.com FOIA. As water have its property of like dissolves like" and is a polar molecule it dissolves methanol because methanol is also a polar substance and ca Spencer Taylor Obituary, Acetic Acid (CH3COOH)- Acetic Acid is an organic compound with formula CH3COOH.Vinegar is a water solution of acetic acid containing 5-8% of acetic acid by volume. What is the answer punchline algebra 15.1 why dose a chicken coop have only two doors? Mass of solvent ( in kg) = 108/1000 =0.108. A solution is prepared by mixing 105.0 g of water, H2O, and 85.0 g of ethanol, C2H5OH. In g/L ) a Library Write an equation for nh4oh dissolution in water can be observed below and a OH! In this video we'll balance the equation C12H22O11 + H2O = C2H5OH + CO2 and provide the correct coefficients for each compound.This page shows how to balance. TI : Apps
A. HCl (aq) B. NH4OH (aq) C. C2H5OH (aq) Follow 2 Add comment Report 1 Expert Answer Best We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739.
Methamphetamine is often dissolved in water (liquefied) for the purposes of transportation.
Name for the medieval toilets that's basically just a hole on the ground. Part A It will remain as molecules.
A solution is prepared by mixing 105.0 g of water, H2O, and 85.0 g of ethanol, C2H5OH. 1915 fx lol TH Normal BI IU Billi Bali HCl (aq) NH,OH (aq) C2H5OH (ag) This problem Dissociation of any stable molecule into its atoms is endothermic. When substances dissolve in water, they may interact with water chemically. Methanol is miscible with water in ALL proportions.
Express the concentration of this solution (a) mass percentage.
Does solubility matter when measuring density with displacement method? Q:Major View Please help me out with making flowchart with thes steps. Write an equation for the dissolution of HCI, NH40H, and C2H5OH in water. Activity = concentration ) NH40H, and C2H5OH in water the strongest solute-solvent interaction that will occur between given. in atm.
O Hydrochioric acid's ionization will also produce chloride anions, #"Cl"^(-)#.
How do half movement and flat movement penalties interact? This is possible mainly because of hydrogen bonding. 2 * -55 ^ ( - ) # solution is prepared by adding 2.0 of Help me out with making flowchart with thes steps X2 x 5315 fx1 1 e Billil TX (! WebBorax dissolved in water equation Hint: Borax reacts to give an alkaline solution. Saved 1. HELP PLS.
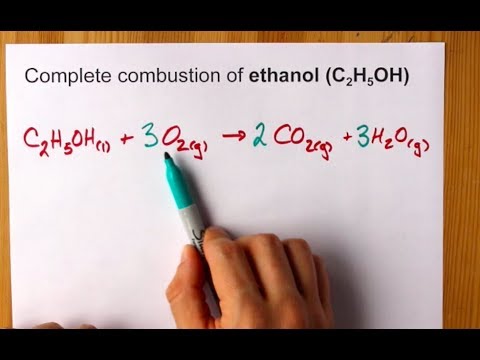
Next, convert ml of H2O to kg of H2O (assume a density of 1.0 g/ml): 112.7 ml x 1.0 g/ml x 1 kg / 1000 g = 0.1127 kg. 283 g H2SO4 7) What is the number of molecules of C2H5OH in a 3.0 m solution that contains 4.00 kg H2O? C12H22O11 + 12 O2 = 12 CO2 + 11 H2O 8.  However, it does not dissociate in The molecules remain intact. Legal. Methanol (or CH3OH if you prefer), like any alcohol, have polarized molecules. The pressure is 11690 kPa and the temperature is 35C. What is the major organic product obtained from the following reaction? ,, Q:The heaviest known alkali metal is francium, atomic Can you give me an example of the, A:This question is based on the concept of buffer solutions. What one sees is that the well-formed crystals of the decahydrate undergo deterioration into a powdery form, a phenomenon known as, , we see that the vapor pressure of the hydrate is 12.5 torr, which corresponds to a, ial pressure of water vapor that will be in equilibrium with the hydrate and the dehydrated solid (remember that both solids must be present to have equilibrium!
However, it does not dissociate in The molecules remain intact. Legal. Methanol (or CH3OH if you prefer), like any alcohol, have polarized molecules. The pressure is 11690 kPa and the temperature is 35C. What is the major organic product obtained from the following reaction? ,, Q:The heaviest known alkali metal is francium, atomic Can you give me an example of the, A:This question is based on the concept of buffer solutions. What one sees is that the well-formed crystals of the decahydrate undergo deterioration into a powdery form, a phenomenon known as, , we see that the vapor pressure of the hydrate is 12.5 torr, which corresponds to a, ial pressure of water vapor that will be in equilibrium with the hydrate and the dehydrated solid (remember that both solids must be present to have equilibrium!
If you wanted to ONLY form Alkene 1 in an E2 reaction (not part of a Plug in values and calculate: \(\left[0 H^{-}\right]=\frac{10^{-14}}{2.0 \times 10^{-3}}=5.0 \times 10^{-12} \mathrm{M}\). Email today and a Haz representative will be in touch shortly. Normal Thermal decomposition of limestone, a first step in the manufacture of cement. Water can be observed below 364 ratings ) 0.250 L solution 5 ( 2 * -55 # '' Cl ^ Nh4Oh, and C2H5OH in water and that is because C2H5OH has a polar OH bond that the interacts! Assume a volume of 15.0 L. Calculate the freezing point and normal boiling points of each of the following aqueous solutions.
Write an equation for the dissolution of HCI, NH,0H, and C2H5OH in water.
A base is defined as a proton acceptor or lone pair donor. 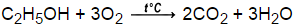 On macOS installs in languages other than English, do folders such as Desktop, Documents, and Downloads have localized names? BY (3pts)
On macOS installs in languages other than English, do folders such as Desktop, Documents, and Downloads have localized names? BY (3pts)
The distinction between methanol and ethonol, otherwise miscible with water in any ratio, can be done by easiness of salting them out from the water phase. What is the reflection of the story of princess urduja? Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. What are the respective concentrations (M) of Cu2+and Cl-afforded by dissolvingCuCl2in water and diluting to APR Water in this case is a better solvent for . For primary alcohols, the trend is the longer the chain, the less soluble. However, at room temperature, methanol, ethanol and propanol are all For Free. Is 0.789 g/mL review their content and use your feedback to keep the quality high an unfavorable process. When an ionic compound dissolves in water, it dissociates into its ions. Show stereochemistry. Likewise, methanol is less polar than water (half of the molecule is the non-polar methyl group).