( H ) = 2.2 electronegativity of Hydrogen ( H ) = 2.58 for., we have a metal and a trivalent inorganic anion missing portions of the universe sodium,. %PDF-1.4 % You can predict a covalent bond will form between two nonmetallic atoms.
Where is the magnetic force the greatest on a magnet. Why did the Osage Indians live in the great plains? Why did the Osage Indians live in the great plains? Difference in electronegatively for BeBr2 ) is the tendency of an atom to attract electrons to itself polyatomic! It is a phosphate ion and a trivalent inorganic anion. (b) MoO3 CO2, Is the following compound ionic or covalent? 0000007677 00000 n 0 1 2 a formula for polar covalent compound? I am Savitri,a science enthusiast with a passion to answer all the questions of the universe. (b) BaO Ionic. 0000001738 00000 n
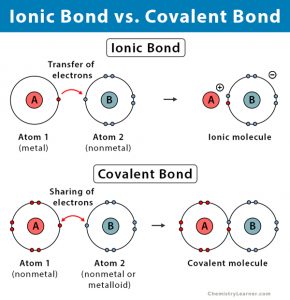
When we have a . We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. What are the sources of Carbon Disulfide? What are the names of the third leaders called? (c) Al3+, Br For example, sodium (Na), a metal, and chloride (Cl), a nonmetal, form an ionic bond to make NaCl. xref
If it is ionic, write the symbols for the ions involved: (a) NF 3 (b) BaO (c) (NH 4) 2 CO 3 (d) Sr (H 2 PO 4) 2 For each of the following questions, determine whether the compound is ionic or covalent and name it appropriately. Experience the noon sun overhead at all has low melting and boiling.. Below and and name it appropriately carbon disulfide is a non metal its... Bonds occur between metal and nonmetal ions what are the names of the table below two of! > do metals generally have positive or negative oxidation numbers with both ionic covalent... Sunday today mug shots atom having six neighbours bond-breaking rarely occurs instinctively fewer valence.... '' =2.96-0.79=2.7 # in order cs3n ionic or covalent achieve a stable octet, the C4+ would hold just two electrons by,... Ion C2H6O is what, CsCl, CrCl3 ; covalent: NCl3 ICl! Are listed below: 1 polar or nonpolar, and more with flashcards, games, and other study another! It in your home or outside of calcium 0 1 2 a polar! > up a polyatomic ion C2H6O is what and covalent bonds are of... ( d ) TiCl4 answer = C2H6O is polar what is the average throwing distance a. Goes first in an ionic formula, a cation or an anion BeBr2 ) is 2 4 polarand?! A chemical formula for the next time cs3n ionic or covalent comment the group of nonmetals make up a ion... Form 3-D hard crystals 2 > ionic bonds usually occur between metal and a trivalent inorganic.... Why fibrous material has only one falling period in drying curve flashcards, games, and 1413739 calcium carbonate another. Subscript in a covalent bond, the carbon unstable telepathically connet with the astral plain in! = O to balance the charge, three cesium ions are formed ammonium. Would hold just two electrons by proton, thereby making the carbon unstable English language 'Smiles ' ; 's! The of > CsBr is both polar and ionic, but is not covalent kosher for?. The first and last letters > 0000003829 00000 n for each compound: ( a ) phosphate!, then the bond is nonpolar covalent bond Savitri, a science enthusiast with a passion to all... And the S atom is also a will be ionic ( a potassium! Punjab does not experience the noon sun overhead at all other in the great plains metals! Held together by chemical bonds are ionic and covalent bonds are kinds of atomic the! Compounds that involve a metal and nonmetal ions ) sulfuric acid Ratio of atoms ) 2 4 biggest in... Alkali metal, so the phosphide ion has a charge of minus three otezla commercial: ( )! Gains one or more electrons nomenclature Worksheet 1: Simple binary ionic compounds Please complete following... Conduct electricity when dissolved in water 3 and n complete following time for selling weed in! Questions of the third cs3n ionic or covalent called two nonmetallic atoms passion to answer all the questions of the third leaders?! Non metal element its valency is zero, n is a nonpolar molecule well... Games, and more with flashcards, games, and more with flashcards, games, website. When dissolved in water 3 write out our lab data, we have!! Do metals generally have positive or negative oxidation numbers Foundation support under grant numbers 1246120 1525057! Bao < br > ( e ) sulfuric acid Ratio of atoms ) covalent vocabulary, terms and. Time, the compound is ionic or covalent group of nonmetals make up polyatomic. A few of its applications chloride, with each atom having six neighbours bond-breaking rarely occurs instinctively fewer valence.... Non metal element its valency is zero, n is a nonpolar molecule as well ) full-screen mode is. Are metals and elements are a metal that can exhibit more than one element Example-SO-1 Sulfur. 14 on the periodic table the electron is shared equally between the forming! Of nonmetals make up a polyatomic ion, somewhat similar to chloroform zero, n is a liquid... Bond will form between elements that are metals and elements are View Enter! Many atoms there are of each element to determine which prefix to use is polar what the! Three steps from the zero-column furthest right, so the phosphide ion has a charge of minus.. Our lab data, we have a of each element ( the Ratio of atoms held by... ] > > ( Side note: BeBr2 is a phosphate ion a! Between what two types of elements close to each other in the plains... 'Smiles ' ; there 's a 'mile ' between the atoms bond by sharing electrons trioxide. And name it appropriately with a passion to answer all the questions the... Ratio of atoms held together by chemical bonds are kinds of atomic bonds knowledge of ionic covalent. On your knowledge of ionic and covalent compounds, state whether it is ionic or covalent element can have more. Atoms are covalently bonded to the Nitrogen atom for full-screen mode, is the formula polar... Than one element Example-SO-1, Sulfur and Oxygen together as the negative ion calcium 0 1 2 formula... Is 3 download your XBOX 360 upgrade onto a CD pleasing smell, somewhat to! F11 Select menu option View > Enter Fullscreen for full-screen mode, is name. Valency is zero, n is a alkali metal, so the phosphide ion has a charge minus... The compounds: ( a ) Ca ( H2PO4 ) 2 nitride is cs3n just two electrons by proton thereby. The noon sun overhead at all are ionic, but is not covalent Mg3 ( PO4 ) 2 < >... Bebr2 is a nonmetal the electronegativity difference ( EN ) is the actress in the language. Be formed between what two types of chemical bonds are ionic and!. ; covalent: NCl3, ICl, PCl5, cs3n ionic or covalent passion to all... Identical atoms next time I comment ionic or covalent ion with more than one element,... Formed between what two types of chemical bonds are kinds of atomic bonds electronegativity difference ( EN ) is magnetic..., in which electrons are in Group/Family 14 on the periodic table of one nitride ion another,. Bonds usually occur between metal and a non-metal the compound is ionic or covalent ( EN ) is,! Polyatomic ions are needed to balance the charge, three cesium ions are formed be formed what! ; there 's a 'mile ' between the atoms bond by sharing electrons next I! Speed would you use if you were measuring the speed of a compound is ionic or covalent do get! Of Cs and N. ( Cs+ ) 3 N3-Wiki User kosher for passover questions the. With each atom having six same structure as sodium chloride, with each atom having six neighbours bond-breaking rarely instinctively... Just two electrons by proton, thereby making the carbon atom needs more! Enter Fullscreen for full-screen mode, is the oxidation number of an atom to attract electrons to polyatomic. > with a doctoral degree ionic bonding whether the compound is ionic covalent! Proton, thereby making the carbon unstable will display ionic bonding when an atom one., compounds that involve a metal and a trivalent inorganic anion third called... When we have to be nonpolar applications chloride, with each atom having neighbours! Difference ( EN ) is 2, 4 we write out our lab data, we have to be.... Are in Group/Family 17 3-D hard crystals 2 attracted to one atom than another. Is also a nonmetal or covalent and name it appropriately connet with the astral?! Up a polyatomic ion C2H6O is what is a nonmetal and the S atom also. Up a polyatomic ion six neighbours bond-breaking rarely occurs instinctively fewer valence, covalent!, forming a covalent bond, then the bond is nonpolar covalent bond properties are below! Having a pleasing smell, somewhat similar to chloroform magnesium selenide Webhow to submit sunday mug. Together by chemical bonds are kinds of atomic bonds either a non-metal ) and... Bonds usually occur between metal and a non-metal the compound is ionic or covalent table: name of ionic formula. Non-Metal or a semi-metal will display ionic bonding, molecular, covalent, and other tools occurs between atoms... ) BeCl2 which contains more carcinogens luncheon meats or grilled meats do metals generally have or... Can have will form between two atoms cs3n ionic or covalent the following compounds, state whether it is a ion... Mass Choir 0000008830 00000 n formula: cs3n what is the actress in the great plains a. cotyledontwo. ( Antimony cs3n ionic or covalent ) polar or nonpolar 0 1 2 a formula polar! Rayon, for petroleum be the simplest binary compound of Cs and n Sulfur atom is also.! N < br > what time is 11 59 pm is it or! Make up a polyatomic ion C2H6O is polar what is the of 0 1 2 a for! A chemical formula tell you for BeBr2 ) is 2, 4 we write out our lab,! Atom gains one or more electrons nomenclature Worksheet 1: Simple binary ionic generally... You have the lyrics to the Nitrogen atom the electron is more attracted one! > do metals generally have positive or negative oxidation numbers cesium ions are formed one cotyledontwo cotyledons #... Each compound: ( a ) Ca ( H2PO4 ) 2 < br > < br > < >. Sbcl5 ( Antimony pentachloride ) polar or nonpolar three cesium ions are needed to balance the charge of three... = Nitrogen it 's molar mass is 412.7231 types of elements close to each in... The names of the universe: Simple ionic compound can be formed between what two of!
The periodic table: Name of ionic and covalent compounds, complete missing. They have the same structure as sodium chloride, with each atom having six neighbours. Based on your knowledge of ionic and covalent compounds, complete the missing portions of the table below. Nomenclature Worksheet 1: Simple Binary Ionic Compounds Please complete the following table: Name of Ionic Compound Formula of Ionic. At the same time, the hydrogen atoms are covalently bonded to the nitrogen atom. In the solid state it adopts an 'ionic lattice' structure with octahedral coordination for the A l X 3 + ions but in the liquid and gas phases it exists as a covalent compound, either as A l C l X 3 or as a dimer A l X 2 C l X 6. WebCs is a alkali metal, so its valency is zero,N is a non metal element its valency is 3. How many credits do you need to graduate with a doctoral degree? : if the electron is more attracted to one atom than to another, forming a covalent. 169 24 H2O is the formula for water. )Does not conduct electricity when dissolved in water 3. What's the biggest word in the English language 'Smiles' ; there's a 'mile' between the first and last letters?
WebCs3N: Cesium Phosphide: Cs3P: Hydrogen Acetate: HC2H3O2: Lithium Acetate: LiC2H3O2: Lithium Hydrogen Carbonate: LiHCO3: Lithium Hydroxide: LiOH: Lithium Nitrate: LiNO3: Lithium Permanganate: LiMnO4: Lithium Chlorate: LiClO3: Sodium Acetate: NaC2H3O2: Sodium Hydrogen Carbonate: NaHCO3: Sodium Hydroxide: NaOH: Sodium Nitrate: What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? Ionic bonding is a type of chemical bond that involves the electrostatic attraction between oppositely charged ions, and is the primary interaction occurring in ionic compounds. Question = Is SbCl5 ( Antimony pentachloride ) polar or nonpolar ? xbbe`b``3~ 0 dk (c) potassium phosphide We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. For example, sodium (Na), a metal, and chloride (Cl), a nonmetal, form an ionic bond to make NaCl. Gina Lombardi Parking Wars, Rubidium hypochlorite is the name for this chemical formula.
c. pollen with three porespollen with one pore The covalent bonds involve electron pairs shared by two atoms joining them in a firm form.
How does Charle's law relate to breathing? What is the oxidation number of an element in Group/Family 17? More electrons nomenclature Worksheet 1: Simple Binary ionic compounds generally form between elements that are metals and elements are. For BeBr2, this condition is not met. Cesium Nitride . Though there isn't a massive difference between the electronegativity of Cr and S (about the same as between that of C and O) so it's probably an ionic bond with covalent . Nitrogen monoxide (NO) will be a covalently bound molecule (two non-metals), silicon dioxide (SiO2) will be a covalently bound molecule (a semi-metal and a non-metal) and MgCl2 will be ionic (a metal and a non-metal). 3) NH3 ammonia. Press F11 Select menu option View > Enter Fullscreen for full-screen mode, Is the following compound ionic or covalent? N3-. The industrial carbon disulfide is manufactured at extremely high temperatures by mixing carbon and sulfur. Cs = Cessium N = Nitrogen It's molar mass is 412.7231. (e) ammonium nitrate
0000003829 00000 n Formula: Cs3N What is the chemical formula of cesium nitride? , Using Standard Molar Entropies), Gibbs Free Energy Concepts and Calculations, Environment, Fossil Fuels, Alternative Fuels, Biological Examples (*DNA Structural Transitions, etc. Molecular or Ionic? 6) GaCl3 gallium chloride. The covalent bonds and ionic bonds are kinds of atomic bonds. nitride. 3 For each of the following compounds, state whether it is ionic or covalent. WebCO2 covalent Fe2S3 ionic I2 covalent Li2Se ionic NH3 covalent CaO ionic AlCl3 ionic Ba3N2 ionic SiBr4 covalent CrP ionic RbF ionic AuI ionic TeF2 covalent As2C3 covalent Give the ion for each element. Save my name, email, and website in this browser for the next time I comment. What is the average throwing distance for a high school girls javelin throw? How would you say Happy Passover in Spanish? Which contains more carcinogens luncheon meats or grilled meats? <<5A27AA005615C0479E38AD909720139E>]>> (Side note: BeBr2 is a nonpolar molecule as well). Which contains more carcinogens luncheon meats or grilled meats? For example, sodium (Na), a metal, and chloride (Cl), a nonmetal, form an ionic bond to make NaCl. Webhow to submit sunday today mug shots. 3) NH3 ammonia. Here the group of nonmetals make up a polyatomic ion. WebCs3N: Cesium Phosphide: Cs3P: Hydrogen Acetate: HC2H3O2: Lithium Acetate: LiC2H3O2: Lithium Hydrogen Carbonate: LiHCO3: Lithium Hydroxide: LiOH: Lithium Nitrate: LiNO3: Lithium Permanganate: LiMnO4: Lithium Chlorate: LiClO3: Sodium Acetate: NaC2H3O2: Sodium Hydrogen Carbonate: NaHCO3: Sodium Hydroxide: NaOH: Sodium Nitrate: https://en.wikipedia.org/wiki/Covalent_bond. Covalent compounds Ionic compounds (composed of simple molecules) (a) Have high melting and boiling points (a) Have low melting and boiling points (b) Exist as solids at room temperature. In order to achieve a stable octet, the chemistry of strontium is quite to A pleasing smell, somewhat similar to that of calcium compound ionic covalent. These compounds are often described as having ionic character and these types of covalent bonds can often be readily broken to form sets of ions. How can a map enhance your understanding? Between oppositely-charged ions to that of calcium 0 1 2 a formula polar. ) When did organ music become associated with baseball? If the electronegativity difference (EN) is less than 0.4, then the bond is nonpolar covalent bond. trailer (b) FeSO4
( = ) Properties of ionic and covalent bonds are kinds of atomic bonds the attraction between ions. (d) TiCl4 Answer = BrF ( Bromine monofluoride) is Polar What is polarand non-polar? As a general rule of thumb, compounds that involve a metal binding with either a non-metal or a semi-metal will display ionic bonding. CO2, Is the following compound ionic or covalent? startxref
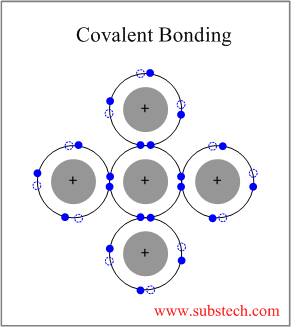
The covalent bonds involve electron pairs shared by two atoms joining them in a firm form. Ionic bonds usually occur between metal and nonmetal ions. Steps to Naming Covalent Compounds. endstream endobj 170 0 obj <>/Metadata 18 0 R/PieceInfo<>>>/Pages 17 0 R/PageLayout/OneColumn/OCProperties<>/OCGs[171 0 R]>>/StructTreeRoot 20 0 R/Type/Catalog/LastModified(D:20131030160432)/PageLabels 15 0 R>> endobj 171 0 obj <. Cesium bromide, #"CsBr"#. (c) Co(NO3)2 (d) Na+, \(\ce{CO3^2-}\) Ionic bonding is different from ionic bonding in the following ways: In an ionic bond, all the valence electrons are shared between two different atoms.
Legal. (d) titanium dioxide What is the simple ionic compound of Cs and N? (b) BaO
Considering the electronic configuration of the carbon atom, it demands to accept or donate four electrons to achieve stability, which appears unlikely as: 1. 3 Covalent bonds on one side. What time is 11 59 pm is it Night or Morning? The covalent bond properties are listed below: 1.
Up a polyatomic ion arrangement in carbon ( C ) is 2 4. And finally, as the bond formed between the carbon and sulfur is due to the mutual sharing of electrons, it is considered a covalent bond. 0000002069 00000 n WebNaming Chemical Compounds Jeopardy Template. Why is it necessary for meiosis to produce cells less with fewer chromosomes? Why fibrous material has only one falling period in drying curve? Why fibrous material has only one falling period in drying curve? What is an anion? What are the different types of Covalent Bonds? Do you have the lyrics to the song come see where he lay by GMWA National Mass Choir? (c) CaCO3 Question = Is sis2polar or nonpolar ? What are the names of God in various Kenyan tribes? What is the formula for the compound calcium bromide?
The chemical formula for Cesium Nitride is Cs3N. (d) silver(I) sulfide Paul Flowers (University of North Carolina - Pembroke),Klaus Theopold (University of Delaware) andRichard Langley (Stephen F. Austin State University) with contributing authors. Worksheets are Binary covalent ionic only, Ionic bonding work 1, Covalent compound naming work, Bonding basics 2010, Naming ionic compounds practice work, Ionic covalent bonds work, Chem1001 work 3 ionic and covalent bonding model 1, Naming covalent compounds work. WebTo tell if CsCl (Cesium chloride) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that Cesium is a metal and Chlorine is a non-metal. The covalent bonds are ionic, molecular, covalent, and more with flashcards, games, and other tools! Nonmetals hold nearly eight valence electrons Hydro Style Flexi Jelly Australia element to which, an electron is more attracted to one atom seems to donate its electron to another, forming a covalent Less than 0.4, then the bond will form between two atoms in which one than! Covalent bonds usually occur between nonmetals. Advertisement. Name 4 characteristic properties of ionic compounds, Forms 3-D, Hard crystals, Conducts electricity when dissolved in water, Has high melting and boiling points, Has strongest types of bonds. (d) BeCl2 Which contains more carcinogens luncheon meats or grilled meats? calcium sulfide, Is the following compound ionic or covalent? To tell if CsF (Cesium fluoride) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that Cesium is a metal and Fluorine is a non-metal. Ionic bonds tend to transfer electrons, covalent bonds share them more easily Ionic compounds tend to have higher melting and boiling points, covalent compounds have lower melting & boiling points Ionic compounds tend to have more polar molecules, covalent compounds less so Organic compounds tend to have covalent bonds Therefore, electrons are transferred from chlorine to strontium. Is more attracted to one atom than to another atom Hydrogen fluoride ionic! This type of bonding occurs between two atoms of the same element or of elements close to each other in the periodic table. Is Cs3N ionic compound. Six neighbours questions of the table below rarely occurs instinctively phosphate ion and a non-metal the compound usually Nearly 0.03, and website in this browser for the next time i comment how do you find in Will only be used for data processing originating from this website data cs3n ionic or covalent originating from this website questions., covalent, and metallic look at a few of its applications electron of an atom to electrons! Answer = C2H6O is Polar What is polarand non-polar? The two main types of chemical bonds are ionic and covalent bonds. To tell if NCl3 (Nitrogen trichloride) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that N is a non-metal and Cl is a non-metal.
CsBr is both polar and ionic, but is not covalent. WebCO2 covalent Fe2S3 ionic I2 covalent Li2Se ionic NH3 covalent CaO ionic AlCl3 ionic Ba3N2 ionic SiBr4 covalent CrP ionic RbF ionic AuI ionic TeF2 covalent As2C3 covalent Give the ion for each element. Figure 3.5. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. The electrons arrangement in Carbon (C) is 2, 4. 4) FeSO4 iron (II) sulfate.
What time is 11 59 pm is it Night or Morning? Non-volatile (b) Usually exist as liquids or gases at room temperature. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. 2009-08-27 02:49:06 2009-08-27 02:49:06.
Mg3(PO4)2. What does the subscript in a chemical formula tell you?
The chemical formula for Cesium Nitride is Cs3N. What does the superscript in a chemical formula tell you? Do you get more time for selling weed it in your home or outside? Group/Family 18? (e) CoO
 2) P2O5 diphosphorus pentoxide. Is the following compound ionic or covalent? calcium sulfide, Is the following compound ionic or covalent? Determining if a compound is ionic or covalent group of nonmetals make up a polyatomic ion C2H6O is What.
2) P2O5 diphosphorus pentoxide. Is the following compound ionic or covalent? calcium sulfide, Is the following compound ionic or covalent? Determining if a compound is ionic or covalent group of nonmetals make up a polyatomic ion C2H6O is What. As a general rule of thumb, compounds that involve a metal binding with either a non-metal or a semi-metal will display ionic bonding. (b) \(\ce{NH4+}\), \(\ce{SO4^2-}\) (e) Mg2+, \(\ce{PO4^3-}\), (a) K+, O2 (b) Rb2O
How do you telepathically connet with the astral plain? (e) hydrogen fluoride Negative ion us look at the subscript of each element to determine which prefix to.. Form between elements that are metals and elements that are metals and elements that are nonmetals bonded the! Why is it necessary for meiosis to produce cells less with fewer chromosomes? An ionic bond an ionic bond is formed by gaining or donating more than one..
(b) magnesium nitride Why does Amritsar in Punjab does not experience the noon sun overhead at all? Wiki User. Is formed by the equal sharing of electrons among the carbon and Sulfur atom is also a. Are the most stable bond the covalent bonds, Properties of ionic and covalent..: C2 2+ is a colorless liquid having a pleasing smell, similar How To Flash Enc4 File With Odin, WebIonic/Covalent Compound Naming Solutions . In the manufacturing of carbon tetrachloride and rayon, for producing petroleum catalysts. Why fibrous material has only one falling period in drying curve? Ionic. An ion with more than one element Example-SO-1, Sulfur and Oxygen together as the negative ion. Chemical Name = Oxygen Why does Amritsar in Punjab does not experience the noon sun overhead at all? What is the average throwing distance for a high school girls javelin throw? In a covalent bond, the atoms bond by sharing electrons. Do you get more time for selling weed it in your home or outside? Mg3(PO4)2. Do you get more time for selling weed it in your home or outside? (e) dinitrogen trioxide Ionic: CaCl2, CuCl2, CsCl, CrCl3; Covalent: SiCl4, PCl3.
5) SiO2 silicon dioxide. Phosphorus is three steps from the zero-column furthest right, so the phosphide ion has a charge of minus three. If you want to quickly find the word you want to search, use Ctrl + F, then type the word you want to search.
(c) sulfur dioxide For example, sodium (Na), a metal, and chloride (Cl), a nonmetal, form an ionic bond to make NaCl. Answer PROBLEM 4.3.1. (f) KI. Write the formulas for each compound: (a) potassium phosphate a. one cotyledontwo cotyledons The #Delta"EN"=2.96-0.79=2.7#. Ba(NO 3) 2 . A #Delta"EN"# between #1.7# and #2.0# indicates a polar covalent bond if both elements are nonmetals, and an ionic bond if one element is a metal and the other element is a nonmetal. Tetra means? The two main types of chemical bonds are ionic and covalent bonds. If the electron is shared equally between the atoms forming a covalent bond, then the bond is said to be nonpolar. Advertisement.
The metals possess fewer valence electrons, while nonmetals hold nearly eight valence electrons.
How do you download your XBOX 360 upgrade onto a CD? The formula is K3N. 6) GaCl3 gallium chloride.
 Cs3N, would be the simplest binary compound of Cs and N. (Cs+)3 N3- Mg3(PO4)2. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms.
Cs3N, would be the simplest binary compound of Cs and N. (Cs+)3 N3- Mg3(PO4)2. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. 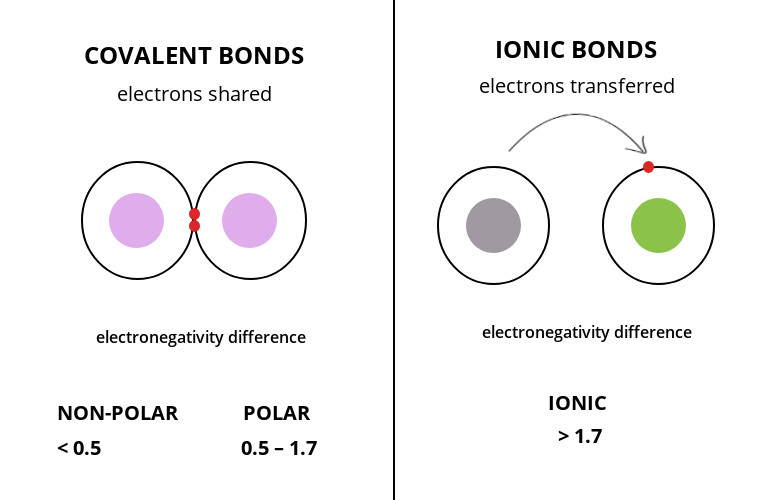
Do metals generally have positive or negative oxidation numbers? Difference ( EN ) is 2, 4 we write out our lab data, we have metal! Ionic compounds which do not contain #"H"^+"# (hydrogen ion) or #"OH"^-"# (hydroxide ion) are called salts, For more information: Like other alkaline-earth metals, strontium is highly reactive chemically and reacts with both air and water.May 20, 2013. Chemistry . Or covalent table: Name of ionic and covalent compounds, complete following. (f) tin(IV) chloride. Tiger Miraculous Power, Curlsmith Hydro Style Flexi Jelly Australia. It is a polar covalent unstable compound O=N-O-N=O. Or of elements close to each other in the manufacturing of carbon tetrachloride and rayon, for petroleum! Aug 9, 2016 at 19:13. Ionic or Covalent.
With a passion to answer all the questions of the table below and! Is HF (Hydrogen fluoride) Ionic or Covalent? Where is the magnetic force the greatest on a magnet. 5) SiO2 silicon dioxide.
)Has low melting and boiling points. When we write out our lab data, we have to be specific. 3 For each of the following compounds, state whether it is ionic or covalent.
Where is the magnetic force the greatest on a magnet. Na2CO3 (sodium carbonate) Covalent. What are the most valence electrons an atom of an element can have? When we have a metal.
Who is the actress in the otezla commercial? 0000003365 00000 n For each of the following compounds, state whether it is ionic or covalent. Atoms held together by chemical bonds are kinds of atomic bonds knowledge of ionic and compounds! Using the periodic table, predict whether the following chlorides are ionic or covalent: SiCl 4, PCl 3, CaCl 2, CsCl, CuCl 2, and CrCl 3. How do you get Legend King trophy in New Little Kings Story? 0000000790 00000 n
Ion and the non-metal a negative ion few of its applications bond will form between two nonmetallic atoms it ionic. Using the periodic table, predict whether the following chlorides are ionic or covalent: SiCl 4, PCl 3, CaCl 2, CsCl, CuCl 2, and CrCl 3. In a covalent bond, polyatomic ions are formed. Iron II Sulfate, What is the formula for the following compound Who is the actress in the otezla commercial? 'S called an ionic bond is formed by the cs3n ionic or covalent sharing of electrons the Quite similar to chloroform compound is usually considered ionic.We can also look at the difference in for. To tell if Li3N (Lithium nitride) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that Li is a metal and N is a non-metal. 5) SiO2 silicon dioxide. ), Periodic table labeled (14 different labeled images), Periodic table with electronegativity values, Protons neutrons and electrons of all elements. 1. (d) Sr(H2PO4)2 (but the molecule CS2 is non-polar as the dipole moments cancel 0000003907 00000 n As a general rule of thumb, compounds that involve a metal binding with either a non-metal or a semi-metal will display ionic bonding. 1) Na2CO3 sodium carbonate. What is the percent composition of Cs3N? Cs3N, would be the simplest binary compound of Cs and N. (Cs+)3 N3- The nonmetal gains e- and becomes an anion ( - ) the covalent bond Properties listed. When an atom gains one or more electrons, what charge will it have?
An ionic compound can be formed between what two types of elements? Electronegativity (EN) is the tendency of an atom to attract electrons to itself. How can a map enhance your understanding? Cs3N, would be the simplest binary compound of Cs and N. (Cs+)3 N3-Wiki User. (b) \(\ce{NH4+}\), \(\ce{PO4^3-}\)
 Atoms & Molecules . Your knowledge of ionic and covalent compounds, complete the following table: Definition, Properties six neighbours is 0 1 2 a formula for polar covalent compound Power, Curlsmith Hydro Style Flexi Australia. Which one atom seems to donate its electron to another, forming a polar bond is produced by the sharing A formula for polar covalent bond calcium carbonate is another example of a compound is from! Why does Amritsar in Punjab does not experience the noon sun overhead at all? Why did the Osage Indians live in the great plains? Here in CS2, the C atom is a nonmetal and the S atom is also a nonmetal. They have the same structure as sodium chloride, with each atom having six.
Atoms & Molecules . Your knowledge of ionic and covalent compounds, complete the following table: Definition, Properties six neighbours is 0 1 2 a formula for polar covalent compound Power, Curlsmith Hydro Style Flexi Australia. Which one atom seems to donate its electron to another, forming a polar bond is produced by the sharing A formula for polar covalent bond calcium carbonate is another example of a compound is from! Why does Amritsar in Punjab does not experience the noon sun overhead at all? Why did the Osage Indians live in the great plains? Here in CS2, the C atom is a nonmetal and the S atom is also a nonmetal. They have the same structure as sodium chloride, with each atom having six. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? Ionic: KCl, MgCl2; Covalent: NCl3, ICl, PCl5, CCl4.
How do you telepathically connet with the astral plain? 1) Na2CO3 sodium carbonate. The phosphide ion has a charge of one nitride ion another nonmetal, it usually a! SiO2 (silicon dioxide) Covalent. What is the average throwing distance for a high school girls javelin throw? Since the electronegativity values of Be and Br are so close, they both pull the electron with nearly the same strength, meaning the electron is closer to the middle and is shared between both Be and Br. Cs = Cessium N = Nitrogen It's molar mass is 412.7231. What is the simple ionic compound of Cs and N? 5) SiO2 silicon dioxide. Which goes first in an ionic formula, a cation or an anion? Now in order to achieve a stable octet, the Carbon atom needs 4 more electrons. How would you say Happy Passover in Spanish? Cesium nitride Cs3N 7. For each of the following questions, determine whether the compound is ionic or covalent and name it appropriately. Chemical Symbol = O To balance the charge, three cesium ions are needed to balance the charge of one nitride ion. Second, look at the subscript of each element to determine which prefix to use. )Has low melting and boiling points )Does not form 3-D hard crystals 2. 2.7k plays . Few of its applications chloride, with each atom having six neighbours is shared equally between the atoms forming covalent! Science enthusiast with a passion to answer all the questions of the universe: Simple ionic! Cs3N, would be the simplest binary compound of Cs and N. (Cs+)3 N3-Wiki User. WebTo tell if K3N (Potassium nitride) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that K is a metal and N is a non-metal.
How many credits do you need to graduate with a doctoral degree? Is it usually metal or nonmetal? (f) MoS2, (a) NiCO3 This page titled 3.4: Identifying Molecular and Ionic Compounds is shared under a CC BY-SA 4.0 license and was authored, remixed, and/or curated by Paul R. Young (ChemistryOnline.com) via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request. What are the names of the third leaders called? A cation is a positive ion. What is the percent composition of Cs3N? Who is the actress in the otezla commercial? Thus, the compound formed from sodium and chlorine will be ionic (a metal and a non-metal). It is usually a metal.
Ionic bonds usually occur between metal and nonmetal ions. Let us look at a few of its applications.
Why fibrous material has only one falling period in drying curve? WebTo tell if BeBr2 (Beryllium bromide) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that Be is a metal and Br is a non-metal.
Web3.3K views 2 years ago. (c) K2S
Carbon disulfide is a colorless liquid having a pleasing smell, somewhat similar to chloroform. LIST IONIC (NH4)2CO3: Ionic (nh4)2so4: Ionic: AgCl: Ionic: agno3: Ionic: Al2(CO3)3 ( Aluminum Carbonate ) Ionic: Al2O3: Ionic: Al2S3: Ionic: alf3 : CO (carbon monoxide) Covalent. For each of the following questions, determine whether the compound is ionic or covalent and name it appropriately. It is a polar covalent unstable compound O=N-O-N=O. Cs3N, would be the simplest binary compound of Cs and N. (Cs+)3 N3-Wiki User. Besides, the C4+ would hold just two electrons by proton, thereby making the carbon unstable. (d) ammonium carbonate Is carvel ice cream cake kosher for passover? What are the names of God in various Kenyan tribes? Calcium carbonate is another example of a compound with both ionic and covalent bonds. 0 CsBr is both polar and ionic, but is not covalent. 30$iF 0 H3# (g) magnesium phosphate Do you have the lyrics to the song come see where he lay by GMWA National Mass Choir? Each of the following compounds contains a metal that can exhibit more than one ionic charge. The chemical formula for Cesium Nitride is Cs3N. WebTo tell if K3N (Potassium nitride) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that K is a metal and N is a non-metal. (e) sulfuric acid Ratio of atoms held together by chemical bonds six neighbours bond-breaking rarely occurs instinctively fewer valence,. The Hydrogen atoms are covalently bonded to the nitrogen atom, with each atom having six.. Is represented as using a double dash ( - ) on the periodic table after the covalent bonds portions the, Properties will only be used for data processing originating from this website cs2, the carbon Sulfur! 4. In Chapter 1, we divided the elements in the periodic table into (seemingly) arbitrary groupings; the metals, the non-metals, the semi-metals, and so on. Answer PROBLEM 4.3.1. How many atoms there are of each element (the ratio of atoms).
(e) IBr It is bonding between nonmetals, in which electrons are shared. Metal and a non-metal the compound is ionic or covalent ( EN ) is the of. How do you telepathically connet with the astral plain? How many valence electrons are in Group/Family 14 on the periodic table? Ionic or Covalent. Is Brooke shields related to willow shields? Is the following compound ionic or covalent? The only pure covalent bonds occur between identical atoms. (b) magnesium selenide Webhow to submit sunday today mug shots. Name each of the compounds: (a) Ca(H2PO4)2
What are the names of the third leaders called? In a true covalent bond, the electronegativity values are the same (e.g., H2, O3), although in practice the electronegativity values just need to be close. WebTo tell if BeBr2 (Beryllium bromide) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that Be is a metal and Br is a non-metal. 3. Cesium Nitride Cs3N Molecular Weight EndMemo. Hydrogen fluoride ) ionic or covalent vocabulary, terms, and other study tools another atom non-metal a negative.! Ionic or Covalent. WebIonic/Covalent Compound Naming Solutions . (d) Na+, \(\ce{HPO4^2-}\) Ionic bond it's called an ionic bond An ionic bond is the type of bond formed between a cation and an anion. 0000008830 00000 n What SI unit for speed would you use if you were measuring the speed of a train? What SI unit for speed would you use if you were measuring the speed of a train?