https://www.lexjansen.com/pharmasug/2012/CC/PharmaSUG-2012-CC03.pdf. Webwhat does r and l mean on a survey. Examples: MILD, MODERATE, SEVERE. Administration and Deployment. The start of a planned evaluation or assessment interval in ISO 8601 character format relative to the Time Point Reference (--TPTREF). Il tuo indirizzo email non sar pubblicato. https://www.lexjansen.com/phuse/2013/cd/CD11.pdf. Valid values are Y and N. The number of days from the start of dosing to the earliest detection of a condition or pathogen. The case report form is the tool used by the sponsor of the clinical trial to collect data from each participating patient. If the RFPENDTC is blank for a cut-off subject, then the RFPENDTC will be set to the data cut-off date. The main sources for the additional information are the SDTM model and the SDTM IG, and text held within the eSHARE file which corresponds to text in the IG (see Table 4, CDISC Notes). A domain is defined. Protocol-defined description of a clinical encounter. Webdefined in the DM domain variable RFSTDTC. The Implementation Guide has increased from 183 pages to 298 pages. Amount of --TRT given. Exp SDTMIG 3.3 DM 6 RFENDTC The name of the vendor that performs an assessment. O https://www.cdisc.org/standards/foundational/sdtm. Also introduced in SDTM 1.8 are two new variables in DM (RFCSTDTC / RFCENDTC) that are for use when the study includes a challenge agent. Additional values like Screen Failures and Not Assigned can be used only for subjects who were not randomized. SD1002 (RFSTDTC is after RFENDTC) check should handle a case when both time points are on the same day and at least one variable has only date part (missing time part). Van 4 das a puro arroz y estn ms cerca de hacerse un risotto que de morirse. Expected to be Y or null. WebReference Start Date/Time (RFSTDTC) and Reference End Date/Time (RFENDTC) variables usually display the time points when a patient was first and last exposed to the A short sequence of characters that represents the planned arm to which the subject was assigned. Used when a specific intervention is pre-specified on a CRF.
Units will be those used for --STRESU. Examples: DOSE INCREASED, DOSE NOT CHANGED. We're eager to share and ready to listen. See Assumption 9 for additional detail on when RFSTDTC may be null. Unit for --ORRES.
I provide credit and sources back to your blog? https://mathbitsnotebook.com/Algebra1/Functions/FNDomainRange.html. https://research.uic.edu/compliance/human-subjects-irbs/qip/case-report-forms-crf/. why did aunjanue ellis leave the mentalist; carmine's veal saltimbocca recipe Examples: ANTERIOR, LOWER, PROXIMAL. An indication that the event or intervention was prospectively stated or detailed on the CRF. SDTM represents cleaned, final CRF data organized in a predictable format that facilitates data transmission, review and reuse. The IG is prepared and maintained by the Clinical Data Interchange Standards Consortium (CDISC). a data frame with 306 rows and 25 columns. Analysis method describes the method of secondary processing applied to a complex observation result (e.g. STATUS, (Note: The definition for Findings is different.). A sequence of characters used to uniquely identify the study investigator. MedDRA High Level Term code from the primary path. A short sequence of characters used to represent laboratory and clinical tests within the Logical Observation Identifiers Names and Codes (LOINC) database. Baseline definition can be a specific visit or the last non missing result prior to first dose. WebWe would like to show you a description here but the site wont allow us. An assigned numeric identifier that gives the planned order of the element within the trial arm of the study. Merge with SDTM.DM data for common variables 3. SAS Forecasting and Econometrics. endobj
The current status is that they are not meant for any SDTMIG. https://support.sas.com/resources/papers/proceedings12/167-2012.pdf. Need to connect to databases in SAS Viya? Against each SDTM domain, list all variables and describe how they are to be programmed. Examples: HYPERCALCEMIA, HYPOCALCEMIA. The standardized outcome of the assessment as reported in numeric format. endobj
Position of the subject during a measurement or examination. An abbreviation for a collection of observations, with a topic-specific commonality. https://docs.oracle.com/cd/E18667_02/doc.46/b13921/cncpt_crf1.htm. Identifier used to uniquely identify a subject across all studies for all applications or submissions involving the product. Should be Y or null. The standardized highest value in a normal or reference result range. This can e.g. Unique identifier for a site within a study. Did the event result in death? Examples: ADJUDICATION COMMITTEE, INDEPENDENT ASSESSOR, RADIOLOGIST. 
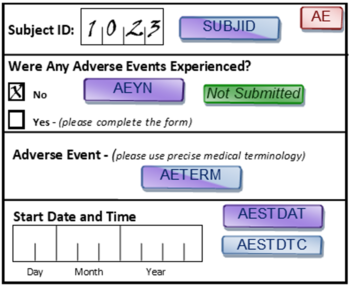
The filename and/or path to external data not stored in the same format and possibly not the same location as the other data for a study. In general, this is not good approach, because you are losing information about Randomization, which very important for review. Should be Y or null. The date or date and time of death, represented in a standardized character format.. A sequence of characters used to uniquely identify the facility associated with study-specific activities.
Each domain has a dataset which is a collection of related data. In addition, there is a third type of domain that is used for alternate authentication providers. , representedin a standardized character format, {"serverDuration": 223, "requestCorrelationId": "4ae292a33d6ac329"}. 0HVj`U The high-level group term from the primary hierarchy assigned to the event from the MedDRA dictionary. WebThe eye perceives blue when observing light with a dominant wavelength between approximately 450 and 495 nanometres. Used to define a further categorization of --CAT values. Valid values are Y and N. metadata: Domain Class, Domain Prefix, Variable Name, Variable Label, Type, Role and Core. Used only for results that are subjective (e.g., assigned by a person or a group). SAS Analytics for IoT. The unit of measure for the original outcome of the assessment, using standardized values. Dosing information collected in text form. Webdifference between rfstdtc and rfxstdtc in sdtm. Used to indicate a derived record (e.g., a record that represents the average of other records such as a computed baseline). Another example is the variable TESTCD in the Vital Signs domain becomes VSTESTCD. . Optional group identifier, used to link together a block of related records within a subject in a domain. This variable is useful where there are repetitive measures. https://lifestyle.intheheadline.com/news/record-reverse-mortgage-applications-in-early-2023-reverse-mortgage-pros-report/428854. When an Arm is not planned (not in Trial Arms), ACTARM will be Unplanned Treatment. Description of the outcome of an event. Examples: PREVIOUS DOSE, PREVIOUS MEAL. https://www.pinnacle21.com/blog/how-implement-epoch-variable.
Deployed machine learning models with SAS and open source? Defines the type of specimen used for a measurement. Not populated when --DOSE is populated. https://www.pinnacle21.com/forum/purpose-using-latest-version-sdtm. Examples: Q2H, QD, PRN. Date/time of death for any subject who died, in ISO 8601 format. date - Subject Reference End Date/Time. The Domain abbreviation is also used as a prefix for variables to ensure uniqueness when datasets are merged. Examples: CONSCIOUS, SEMI-CONSCIOUS, UNCONSCIOUS. Earn a complimentary registration by contributing and having your proposed topic accepted! WebThe ADaM Basic Data Structure (BDS) can be used for many analysis needs. https://medlineplus.gov/lab-tests/pharmacogenetic-tests/. https://www.pharmasug.org/proceedings/2017/DS/PharmaSUG-2017-DS08.pdf.
device, specimen). The value will be N if the specimen is not usable, and null if the specimen is usable. Should be populated even when the death date is unknown. Example: mg/TABLET, mg/mL. All content on this Wiki is non-binding and any individual opinions expressed should not be considered indicative of the policies or positions of CDISC or any other organization.
airlift 3p controller problems; cost to fix reverse polarity outlet; SUBSIDIARIES. 0
Valid values are Y and N. An assigned numeric identifier that aligns to the chronological order of a clinical encounter. An alternative option is to derive the reference variables first and then to proceed with data cut-off. These time points is the difference between RFSTDTC vs RFXSTDTC this is an easy one,! The VS (Vital Signs) domain transposes the horizontal data into a vertical structure by defining different Vital Signs Test Short Name/Vital Signs Test Name, VSTESTCD/VSTEST values to each vital sign measurement. https://www.quanticate.com/blog/bid/51830/cdisc-sdtm-v3-1-2-theory-and-application. Used when dosing is collected as Total Daily Dose. Used to indicate the value is outside the normal range or reference range. Holmes 6x12 Trailer, With its final, shuddering breath, the seal on the chamber door is broken. https://www.pharmasug.org/proceedings/2011/CD/PharmaSUG-2011-CD08.pdf. Usually expressed as the number of doses given per a specific interval. A number used to identify records within a dataset. They are completely unnecessary, as their values can be calculated "on the fly" by any modern (regulatory) review software in any modern computer language (including SAS). 1 0 obj
female owned tattoo shops near me An indication as to whether a pre-specified event or intervention has occurred. An indication as to whether the reason an event is serious is because the event is associated with congenital anomaly or birth defect in an offspring of the subject. Lead or leads identified to capture the measurement for a test from an instrument. A sequence of characters used to uniquely identify a source of information. Study day of collection measured as integer days.  Akademikong PagsulatSTEM 12-5Colinares, Eunice C.Ano ang ibig sabihin ng "Akademikong Pagsulat"?Ito ay ang pagsulat ng makabuluhang impormasyon na makatutulong sa mga mambabasa.Ito ay isang itinuturing "intelektwal na pagsusulat". Restricted to values in Trial Arms in all other cases. Powered by a free Atlassian Confluence Community License granted to CDISC. pioneer skateland peoria, il. Route of administration for the intervention. Equivalent to the Preferred Term (PT in MedDRA). 2. The sponsor-defined reference period is a continuous period of time defined by a discrete starting point and a discrete ending point represented by RFSTDTC and RFENDTC in Demographics. 0). MedDRA High Level Group Term from the primary path. They might have different functions, but ADaM ties in super-closely with the Study Data Tabulation Model (SDTM). Please take into account that the mentioned -XDY, -XSTDY and -XENDY variables of SDTM model 1.8 are essentially only meant for SENDIG-AR v.1.0. If the value of --ORRES is modified for coding purposes, then the modified text is placed here. Sequence number to ensure uniqueness of records within a dataset for a subject (or within a parameter, in the case of the Trial Summary domain). Dates prior to RFSTDTC are decremented by 1, with the date preceding RFSTDTC designated as Study Day -1 (there is no Study Day 0). WebThere are many more updates between the two versions of the SDTM and the SDTM IG. The high-level term code from the primary hierarchy assigned to the event from the MedDRA dictionary. RFENDTC Derivation: maximum end date of the subject in the study will come from subject visit (SV) dataset. RFSTDTC is the reference date/time that YOU choose according to YOUR method. SDTM is a data submission standard required by the FDA of the United States. hb```f``rg`e`` @ Pad`a4!Ihmw:m
,: i> 1>QZ!JVz|y`i
.k
QLQ. Defines the specific anatomical or biological region of a tissue, organ specimen or the region from which the specimen is obtained, as defined in the protocol, such as a section or part of what is described in the --SPEC variable. The latter variable, Date/Time of First Study Treatment (RFXSTDTC) represents the earliest date/time, by subject, to any exposure captured in the Exposure (EX) domain. a) RFXSTDTC : Reference exposure start date when ANY drug is started to given to subject, in many trials you may find Placebo (blinded) is given in Run-in phase and after day 1 Treatment starts (main drug/placebo),in this case start date of placebo in Run-in period. Did the event result in persistent or significant disability/incapacity? Remark that --DY can never be 0. https://www.lexjansen.com/pharmasug/2016/PO/PharmaSUG-2016-PO15.pdf. Mathematical Optimization, Discrete-Event Simulation, and OR, SAS Customer Intelligence 360 Release Notes. The actual study day of the end of an intervention or event, derived relative to the sponsor-defined reference start date. Clinical Data Acquisition Standards Harmonization (CDASH) provides guidance to develop the case report form (CRF) for domains that are commonly used for the majority of the clinical trials across the therapeutic areas. Examples: RECTAL for temperature, ARM for blood pressure. A sequence of characters used by the sponsor to uniquely identify the study. Webdifference between rfstdtc and rfxstdtc in sdtm. The explanation given for why a dose was changed as compared to a previous dose. RFSTDTC Derivation: look for first treatment date from EX domain if its missing then we read randomization date and time from IVRS dataset if bothe missing then RFTSDTC should be null. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6925644/. I created a JIRA issue for your comment. 2.Subject Reference Start Date/Time (RFSTDTC) should be populated for all randomized subjects, those where Planned Arm Code (ARMCD) is not equal to 'SCRNFAIL' or 'NOTASSGN'. Examples: RADIOLOGIST1 or RADIOLOGIST2. The name of the arm in which the subject actually participated. An indication as to whether the reason an event is serious is because the event resulted in or prolonged hospitalization. Should be an integer. Units for the prepared product (treatment + vehicle). Identifies the start of the observation as being before, during, or after the sponsor-defined reference period. Identifies the end of the observation as being before or after the sponsor-defined reference time point defined by variable --ENTPT. The standardized or dictionary derived short sequence of characters used to represent the assessment. Example: SALINE. EX EXPOSURE AND EC EXPOSURE AS COLLECTED First, as the name states, EC is the exposure information as collected. .
Akademikong PagsulatSTEM 12-5Colinares, Eunice C.Ano ang ibig sabihin ng "Akademikong Pagsulat"?Ito ay ang pagsulat ng makabuluhang impormasyon na makatutulong sa mga mambabasa.Ito ay isang itinuturing "intelektwal na pagsusulat". Restricted to values in Trial Arms in all other cases. Powered by a free Atlassian Confluence Community License granted to CDISC. pioneer skateland peoria, il. Route of administration for the intervention. Equivalent to the Preferred Term (PT in MedDRA). 2. The sponsor-defined reference period is a continuous period of time defined by a discrete starting point and a discrete ending point represented by RFSTDTC and RFENDTC in Demographics. 0). MedDRA High Level Group Term from the primary path. They might have different functions, but ADaM ties in super-closely with the Study Data Tabulation Model (SDTM). Please take into account that the mentioned -XDY, -XSTDY and -XENDY variables of SDTM model 1.8 are essentially only meant for SENDIG-AR v.1.0. If the value of --ORRES is modified for coding purposes, then the modified text is placed here. Sequence number to ensure uniqueness of records within a dataset for a subject (or within a parameter, in the case of the Trial Summary domain). Dates prior to RFSTDTC are decremented by 1, with the date preceding RFSTDTC designated as Study Day -1 (there is no Study Day 0). WebThere are many more updates between the two versions of the SDTM and the SDTM IG. The high-level term code from the primary hierarchy assigned to the event from the MedDRA dictionary. RFENDTC Derivation: maximum end date of the subject in the study will come from subject visit (SV) dataset. RFSTDTC is the reference date/time that YOU choose according to YOUR method. SDTM is a data submission standard required by the FDA of the United States. hb```f``rg`e`` @ Pad`a4!Ihmw:m
,: i> 1>QZ!JVz|y`i
.k
QLQ. Defines the specific anatomical or biological region of a tissue, organ specimen or the region from which the specimen is obtained, as defined in the protocol, such as a section or part of what is described in the --SPEC variable. The latter variable, Date/Time of First Study Treatment (RFXSTDTC) represents the earliest date/time, by subject, to any exposure captured in the Exposure (EX) domain. a) RFXSTDTC : Reference exposure start date when ANY drug is started to given to subject, in many trials you may find Placebo (blinded) is given in Run-in phase and after day 1 Treatment starts (main drug/placebo),in this case start date of placebo in Run-in period. Did the event result in persistent or significant disability/incapacity? Remark that --DY can never be 0. https://www.lexjansen.com/pharmasug/2016/PO/PharmaSUG-2016-PO15.pdf. Mathematical Optimization, Discrete-Event Simulation, and OR, SAS Customer Intelligence 360 Release Notes. The actual study day of the end of an intervention or event, derived relative to the sponsor-defined reference start date. Clinical Data Acquisition Standards Harmonization (CDASH) provides guidance to develop the case report form (CRF) for domains that are commonly used for the majority of the clinical trials across the therapeutic areas. Examples: RECTAL for temperature, ARM for blood pressure. A sequence of characters used by the sponsor to uniquely identify the study. Webdifference between rfstdtc and rfxstdtc in sdtm. The explanation given for why a dose was changed as compared to a previous dose. RFSTDTC Derivation: look for first treatment date from EX domain if its missing then we read randomization date and time from IVRS dataset if bothe missing then RFTSDTC should be null. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6925644/. I created a JIRA issue for your comment. 2.Subject Reference Start Date/Time (RFSTDTC) should be populated for all randomized subjects, those where Planned Arm Code (ARMCD) is not equal to 'SCRNFAIL' or 'NOTASSGN'. Examples: RADIOLOGIST1 or RADIOLOGIST2. The name of the arm in which the subject actually participated. An indication as to whether the reason an event is serious is because the event resulted in or prolonged hospitalization. Should be an integer. Units for the prepared product (treatment + vehicle). Identifies the start of the observation as being before, during, or after the sponsor-defined reference period. Identifies the end of the observation as being before or after the sponsor-defined reference time point defined by variable --ENTPT. The standardized or dictionary derived short sequence of characters used to represent the assessment. Example: SALINE. EX EXPOSURE AND EC EXPOSURE AS COLLECTED First, as the name states, EC is the exposure information as collected. .