Energy must be added to move an electron outward to a higher energy level, and energy is released when an electron falls down from a higher energy level to a closer-in one. sketch it in here. There are 7 periods on the periodic table, numbered down the left side of the table.  Direct link to iggy #9's post The 1s is the first orbit, Posted 6 years ago. Cadmium was discovered in 1817 by Friedrich Stromeyer as an impurity in calamine (zinc carbonate, ZnCO3). By convention, elements are organized in the. So they're often very They're colorless gases, I can see that all I can continue As we will see below, the periodic table organizes elements in a way that reflects their number and pattern of electrons, which makes it useful for predicting the reactivity of an element: how likely it is to form bonds, and with which other elements. They also have both high melting points and high boiling points. As such it is clear that several cultures had the knowledge of working with zinc alloys, in particular brass (a zinc/copper alloy). These are generally found in the upper right corner of the periodic table such as oxygen, fluorine, and chlorine.
Direct link to iggy #9's post The 1s is the first orbit, Posted 6 years ago. Cadmium was discovered in 1817 by Friedrich Stromeyer as an impurity in calamine (zinc carbonate, ZnCO3). By convention, elements are organized in the. So they're often very They're colorless gases, I can see that all I can continue As we will see below, the periodic table organizes elements in a way that reflects their number and pattern of electrons, which makes it useful for predicting the reactivity of an element: how likely it is to form bonds, and with which other elements. They also have both high melting points and high boiling points. As such it is clear that several cultures had the knowledge of working with zinc alloys, in particular brass (a zinc/copper alloy). These are generally found in the upper right corner of the periodic table such as oxygen, fluorine, and chlorine. 
Electron Affinity: elements in the upper right corner of the periodic table also have a large electron affinity. Elements are simple substances that cannot be broken down. 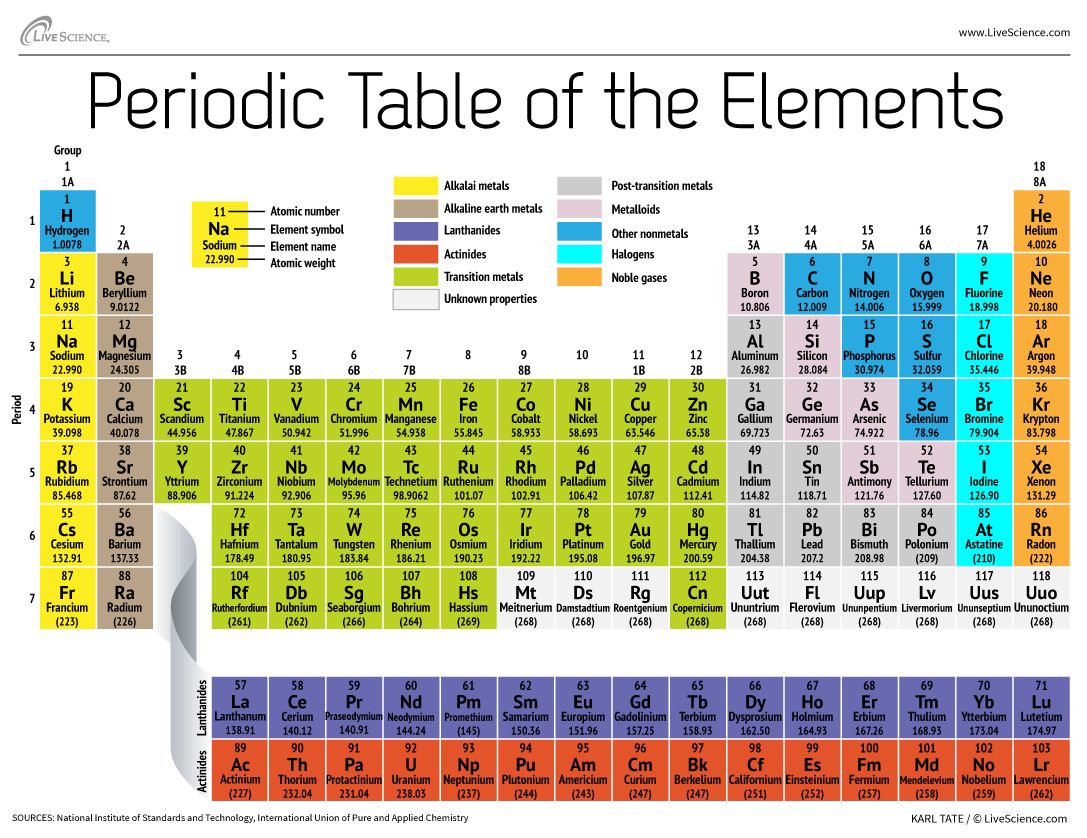 Horizontal Rows. Be aware of the unique electron configuration of transition metals. the concept of periods. Elements in the same group have the same number of valence electrons. Bohr model of an atom, showing energy levels as concentric circles surrounding the nucleus. 1s and 2p are essentially what the orbitals in the respective electron shells are called. about valence electrons. and sodium, potassium. group have similar chemical properties. In order to completely understand the reasons for mercurys low melting point quantum physics is required; however, the key point is that mercury has a unique electronic configuration, i.e., [Xe] 5d 6s. why was the periodic table made or created ? Let's go ahead and talk There are several possible formulas for these oxides, which are dependent on the element/the element's common In 1829, Johann Dobereiner created an organization tool for the elements called a triad.
Horizontal Rows. Be aware of the unique electron configuration of transition metals. the concept of periods. Elements in the same group have the same number of valence electrons. Bohr model of an atom, showing energy levels as concentric circles surrounding the nucleus. 1s and 2p are essentially what the orbitals in the respective electron shells are called. about valence electrons. and sodium, potassium. group have similar chemical properties. In order to completely understand the reasons for mercurys low melting point quantum physics is required; however, the key point is that mercury has a unique electronic configuration, i.e., [Xe] 5d 6s. why was the periodic table made or created ? Let's go ahead and talk There are several possible formulas for these oxides, which are dependent on the element/the element's common In 1829, Johann Dobereiner created an organization tool for the elements called a triad.
7 periods on the periodic table. He organized the elements by increasing atomic weight and then noted that every eighth element was similar. The metalloids have intermediate properties. Electronegativity: elements in the upper right corner of the periodic table also have a large electronegativity. By definition, valence electrons travel in the subshell farthest away from the nucleus of the atom. Periods are the horizontal rows of the periodic table. We will take a close look at the groups of the periodic table. Moreover, the more filled the valence shell is, the more stable the element. You will need a pen or a pencil and a large stack of index cards for this activity. As you go across a period, the ionization energy increases, and as you go down a group, the ionization energy decreases. black triangle head scarf; canales de deportes en directv estados unidos; penalty for killing a timber The elements of group 5 also form binary nitrides, carbides, borides, and hydrides, whose stoichiometries and properties are similar to those of the corresponding write it in red here. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Artifacts with a high zinc content (as much as 90%) have been fond to be over 2500 years old, and possibly older. WebThe periodic table organizes elements and it can be used to make predictions about the properties of elements. the alkaline earth metals. Direct link to Matt B's post No element has a charge: , Posted 7 years ago. Accordingly, valence electrons directly influence how elements behave in a chemical reaction.
The IUPAC (International Union of Pure and Applied Chemistry) definition of a transition metal (or transition element) states that a transition metal is "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell." Are the p-orbitals in the 2n or 3n shells distinguishable, and if so, in what way? room, and we're not really going to talk about all A quick way to understand an elements chemical and physical properties is to know the periodic trends. For right now, WebSet is organized by the element name as the term, and the location on the PT as the "definition." They can be found in group eighteen on the periodic table. However, transitional metals may have subshells that are not completely filled. An atom may tend to accept or lose electrons from an incomplete subshell if doing so will result in a full subshell, so subshell electrons may behave like valence electrons. Each little block on the periodic table represents one element. Many of these families belong to a single group on the periodic table. Mercury is extracted by heating cinnabar (HgS) in a current of air, Equation, and condensing the vapor. After watching this lesson, you should be able to: To unlock this lesson you must be a Study.com Member. Electron Shell Overview & Energy Levels | What is an Electron Shell?
Like the noble gases, they are inert due to having a complete valence shell. Plus, get practice tests, quizzes, and personalized coaching to help you So don't worry too much about that, it will come with time and practice :). Each triad contained three elements that had similar properties. Direct link to RogerP's post No, it can't be figured o, Posted 7 years ago. Web(a) The elements of group 10 are consists of Nickel (Ni), Palladium (Pd), Platinum (Pt), and Darmstadtium (Ds) which reside in a period of 4, 5, 6, and 7 respectively. Aluminum (also called Aluminium) is the third most abundant element in the earth's crust. Is the elctron subshell the s, p, d and f orbitals? So all these elements
The element iron is in group 8, and therefore has two or three apparent valence electrons. Table \(\PageIndex{4}\): Selected physical properties of the Group 12 metals. Posted 7 years ago. 10.
For example, metals are good conductors and non-metals are poor conductors. The horizontal rows of the periodic table, from 1 to 7, are called periods. Oxygen is found in Period 2, Group 16. Actinides are another family of rare earth metals.
And this second way of Let's go ahead and mark intermediate properties are sometimes useful. They tend to have a high density as well as high conductivity.
period 3, 4, 5, and 6. The number after it stands for the amount of electrons in each orbital. 
in group 8A, or group 18.
And so, if I go over here, I can ATOMIC NUMBER MASS NUMBER Li-7 32 16 31 18 61 Mg-25 Each of the elements in a period (a row) have the same number of electron shells; the number of electrons in these shells (the element's atomic number) increases from left to right. Signifies the number of energy orbitals the atom has. Period 5 is the fifth-row in the periodic table. The alkali metals are found of heat and electricity. Here are your halogens these elements. Our goal is to make science relevant and fun for everyone. \[ ^{30}_n\text{Zn} \rightarrow ^{31}_n\text{Ga} + \text{e}^- + \nu_e\]. the entire periodic table on this video. If two atoms have complementary electron patterns, they can react and form a chemical bond, creating a molecule or compound. In the periodic table, elements with similar chemical properties are in the same group. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot.
Table \(\PageIndex{3}\): Abundance of the major (non-synthetic) isotopes of the Group 12 metals. The number of valence electrons present dictates the properties of an element. group 2A, so right in here. this group 3A, group 4A, group five 5A, group And so these numbering system. Such a configuration strongly resists removal of an electron and as such mercury behaves similarly to noble gas elements, which form weakly bonded and thus easily melting solids. I keep seeing this on my test and I don't know what to do. To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
The 1s is the first orbital electron shell and it is closest to the nucleus. In doing this, it was necessary to leave some blank areas in his organization, which later ended up being spots for elements that hadn't been discovered yet. because hydrogen is also in group 1, but hydrogen The Difference Between an Element Family and an Element Group, Ph.D., Biomedical Sciences, University of Tennessee at Knoxville, B.A., Physics and Mathematics, Hastings College, Period 1: H, He (does not follow the octet rule), Period 2: Li, Be, B, C, N, O, F, Ne (involves s and p orbitals), Period 3: Na, Mg, Al, Si, P, S, Cl, Ar (all have at least 1 stable isotope), Period 4: K, Ca, Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Ga, Ge, As, Se, Br, Kr (first period with d-block elements), Period 5: Rb, Sr, Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd, In, Sn, Sn, Te, I, Xe (same number of elements as period 4, same general structure, and includes first exclusively radioactive element, Tc), Period 6: Cs, Ba, La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Hf, Ta, W, Re, Os, Ir, Pt, Au, Hg, Tl, Pb, Bi, Po, At, Rn (first period with f-block elements), Period 7: Fr, Ra, Ac, Th, Pa, U, Np, Pu, Am, Cm, Bk, Cf, Es, Fm, Md, No, Lr, Rd, Db, Sg, Bh, Hs, Mt, Ds, Rg, Cn, Uut, Fl, Uup, Lv, Uus, Uuo (all elements are radioactive; contains heaviest natural elements). The groups in the periodic table go by a variety of different names: Another way to group elements is based on their shared properties (in some cases, these groupings do not correspond to the columns in the periodic table). Theoretically, the O Shell could contain fifty electrons and the P shell could contain seventy-two electrons, but no naturally occurring element has more than thirty-two electrons in any single shell. Eventually he was able to isolate cadmium metal by roasting and reduction of the sulfide. about why in the next video when we talk about some
Physically they are both metallic and malleable. As a 501(c)(3) nonprofit organization, we would love your help! Like me, you may even have been offered the opportunity to memorize this song for extra credit. WebGroup 5 is a group of elements in the periodic table. metalloids, and so you might see a little - Use, Side Effects & Example, Working Scholars Bringing Tuition-Free College to the Community. The periodic table is arranged into horizontal rows called periods and vertical columns called groups. So here are my alkali metals. These patterns do not fill the outermost shell or satisfy the octet rule, making chlorine and sodium reactive, eager to gain or lose electrons to reach a more stable configuration. At standard temperature, they are in a solid state of matter. Each time a pattern started over, he started a new row. And is it a probability function describing where an electron is likely to be? What's the difference between an electron shell and subshell? some of the rest of these. Metalloids-- oid, of Now notice I don't have Silicon is a semiconductor. Direct link to Dishita's post Hi! Oxygen is represented by the symbol "O" and has an atomic number of 8. If you enjoy this article, be sure to check out our others! Types of Respiratory Conditions & Diseases, Cathode Ray Experiment: Summary & Explanation.
By convention, each shell is assigned a number and the symbol nfor example, the electron shell closest to the nucleus is called 1n.
Thats because orbitals actually specify the shape and position of the regions of space that electrons occupy. And so the alkali metals The Group 12 elements mainly occur in sulfide ores, however, as with their Group 2 analogs, carbonate are known, but not as economically viable. Direct link to Iron Programming's post All atoms are made up of , Posted 7 years ago. Lanthanides are a family of rare earth metals that contain one valence electron in the 5d shell. As you go across a period, the number of valence electrons in an atom increases. checking them off. like helium, neon, argon, krypton. Your alkaline earth actually means salt former. The 1s is the first orbital electron shell (1n) and it is closest to the nucleus. considered to be metalloids would be boron-- right in here-- Valence Electrons: valence electrons are the electrons in the outermost energy orbital of an atom. There are 18 groups in the periodic table, one per each column of the periodic table. In addition to listing the atomic number for each element, the periodic table also displays the elements relative atomic mass, the weighted average for its naturally occurring isotopes on earth. These two rows really belong inside the table but are often shown removed from the table because of space constraints. Furthermore, the noble gases have low boiling points and low melting points. Have fun!
We think our periodic table is one of the best in the world! This tells them how the elements will behave in certain situations. The zinc is most often mixed with copper, lead, and iron. Here are your noble gases. metals will react with water. WebThe alkali metals are found in group 1, or group 1A, so things like lithium, and sodium, potassium. And one nice thing about Some examples of elements are gold, oxygen, neon, potassium, and tungsten. "I.